Proteases and Their Control in Health and Disease
Presenter: Robert Huber
Published: July 2014
Age: 18-22 and upwards
Views: 1419 views
Tags: proteolytic;enzyme;catalyse;control;disease;x-ray;diffraction
Type: Lectures
Source/institution: Lindau-Nobel
Watch now
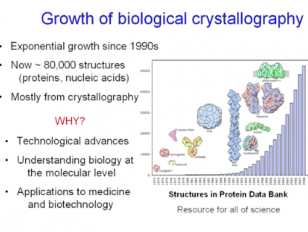
Proteolytic enzymes catalyse a very simple chemical reaction, the hydrolytic cleavage of a peptide bond. Nevertheless, they constitute a most diverse and numerous lineage of proteins. The reason lies in their role as components of many regulatory physiological cascades in all organisms. To serve this purpose and to avoid unwanted destructive action, proteolytic activity must be strictly controlled. Control is based on different mechanisms, which I will discuss and illustrate with examples thereby focusing on intracellular proteases. The regulatory principles seen offer opportunities for therapeutic intervention. The basis of the studies described and of molecular biology in general is X-ray diffraction of crystals, discovered by Laue in 1912 in München. We celebrated the Laue Centennial in the year 2012. With a few slides, I shall commemorate history and the development of Laue’s discovery, which revolutionized many fields of science.